Labeling Errors in Medicinal Products Linked to Poor Packaging Practices
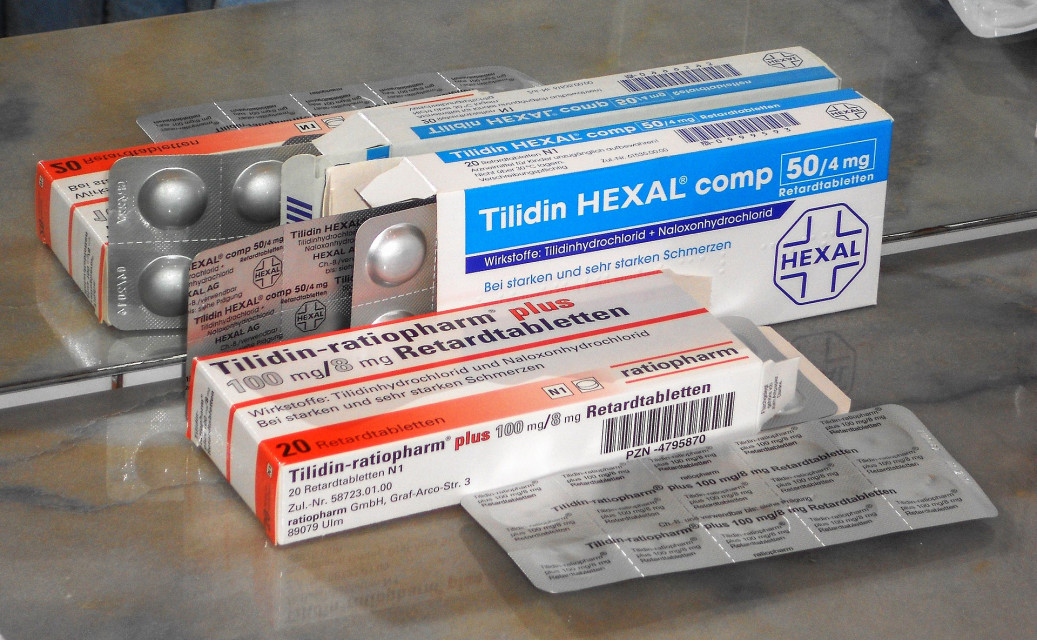
Labeling errors in medicines are often the result of inadequate packaging practices, which stem from weaknesses in the overall quality assurance system. These errors, if not addressed, can have serious consequences for patient safety and may lead to costly drug recalls.
Defective packaging can cause a range of issues, including breakage, problems with printing or inks, and inaccuracies on labels or package inserts (patient information leaflets). These defects compromise the integrity of the product and could pose significant risks to consumers.
To prevent such issues, implementing robust quality control systems is crucial. A strong quality assurance framework ensures that defective medicinal products are not released into the market, safeguarding both public health and the reputation of pharmaceutical companies.
For more information on how to improve packaging and quality control processes, visit this link.
https://www.pharmamicroresources.com/.../how-to-improve...
Credit: Tim Sandle


0 Comments
No Comments available. Be the first to comment on this post
Leave a Comment
Your email address will not be published. Required fields are marked *